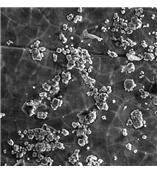
The United States Pharmacopoeia (USP) defines the allowable limits of particulate matter in injections and parenterals in Test Section 788. This section classifies particulate matter in injections and parenteral infusions as any unintentional presence of mobile undissovled particles'' with the exclusion of gas bubbles. The USP sets forth two methods in which to check the amount of particulate matter within an injection: Method 1 is a Light Obscuration Particle Count Test and Method 2 is a Microscopic Particle Count Test.
A microscope is needed in order to perform the Microscopic Particle Count Test'' and it must meet specific requirements. The microscope must have an ocular micrometer that must be calibrated with and objective micrometer. The microscope must have a mechanical stage capable of holding and traversing the whole of the filtration area of the membrane filter. The microscope must have two suitable illuminators'' so that episcopic illumination is provided in addition to oblique illumination. The microscope must be adjustable to 100 ± 10 magnifications.
The restrictions for the tests are such that only a relative error within ± 2% is acceptable for the linear scale of the graticule.
The results for the Method 1 test comply with the standards if the sample tested does not exceed 6000 particles'' on average'' per container that are equal to or greater than 10 micrometers'' and must not exceed 600 particles equal to or greater than 25 micrometers per container. The results for the Method 2 test comply with the standards if the sample tested does not exceed 3000 per container equal to or greater than 10 micrometers'' and does not exceed 300 particles equal to or greater than 25 micrometers per container.

Since such specific limits are set for the allowable undissolved particles'' a precise'' powerful tool is needed to comply with these standards. The ASPEX Rx is the ideal tool for these tests'' being able to quickly and accurately identify foreign particles as small as 0.1 microns. The Rx also is set to perform one click reporting for fast results. Since Rx is designed specifically for the pharmaceutical industry'' it is the clear choice for analysis of metallic particles and the identification of particulates within both drug powders and suspensions.
The Aspex Personal Scanning Electron Microscope (Personal SEM(R)) with the Rx integrated software is the ultimate automated choice for meeting the USP standards set forth in Test Section 788 with ease and accuracy'' built for the pharmaceutical industry with that industry in mind.

Reference:
U.S. Pharmacopoeia'' http://www.usp.org/pdf/EN/USPNF/revisionGeneralChapter788.pdf
Aspex'' http://www.aspexcorp.com/products/rx.html
Aspex'' http://www.aspexcorp.com/industries/health-sciences-reported-info.html